- KYPROLIS® (carfilzomib) is indicated in combination with dexamethasone, or with lenalidomide plus dexamethasone, or with daratumumab plus dexamethasone, ... Read More Close
Kd:
KYPROLIS® + dexamethasone
Superior efficacy in a proteasome inhibitor1,2
Phase 3, randomized, open-label superiority study: (N = 929) comparing Kd 56 mg/m2 twice weekly to Vd in relapsed or refractory multiple myeloma patients who had received 1 to 3 prior lines of therapy. The primary endpoint was PFS. Select secondary endpoints included OS, ORR, DoR, and safety.1,2
CI, confidence interval; DoR, depth of response; Kd, carfilzomib + dexamethasone; HR, hazard ratio; mPFS, median progression-free survival; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; Vd, bortezomib + dexamethasone.
CR, complete response; Kd, carfilzomib + dexamethasone; ORR, overall response rate; PR, partial response; Vd, bortezomib + dexamethasone;
VGPR, very good partial response.
PFS results with Kd were consistent regardless of prior bortezomib exposure1,*
*Study was not powered for PFS efficacy in either subgroup, and estimation of PFS in these subgroups was not a study objective.
CI, confidence interval; HR, hazard ratio; Kd, carfilzomib + dexamethasone; mPFS, median progression-free survival; PFS, progression-free survival;
Vd, bortezomib + dexamethasone.
Study Design
Kd vs Vd in RRMM (ENDEAVOR)
Phase 3, randomized, open-label superiority study: (N=929) comparing Kd 56 mg/m2 twice weekly to Vd in relapsed or refractory multiple myeloma patients who had received 1 to 3 prior lines of therapy. The primary endpoint was PFS. Select secondary endpoints included OS, ORR, DoR, and safety.1,2
Study schema1,2
Key eligibility criteria (N = 929):
Key exclusion criteria:
*Patient stratification included prior proteasome inhibitor therapy (either bortezomib or carfilzomib, or no prior therapy); prior lines of therapy (1 vs 2 or 3); International Staging System stage (1 vs 2 or 3); and planned route of bortezomib administration, if randomized to the bortezomib group (intravenous vs subcutaneous).
†NYHA classification of heart failure III is defined as: Marked limitation of physical activity. Comfortable at rest. Less than ordinary activity causes fatigue, palpitation, or dyspnea (shortness of breath). IV is defined as: Unable to carry out any physical activity without discomfort. Symptoms of heart failure at rest. If any physical activity is undertaken, discomfort increases.
BIW, twice a week; CrCI, creatinine clearance; DoR, depth of response; ECOG PS, Eastern Cooperative Oncology Group Performance Status; Kd, carfilzomib and dexamethasone; LVEF, left ventricular ejection fraction; MM, multiple myeloma; NYHA, New York Heart Association; ORR, overall response rate; OS, overall survival;
PFS, progression-free survival; PI, proteasome inhibitor; PR, partial response; R, randomization; Vd, bortezomib and dexamethasone.
(View data)
Baseline disease characteristics and prior treatments were balanced between study arms
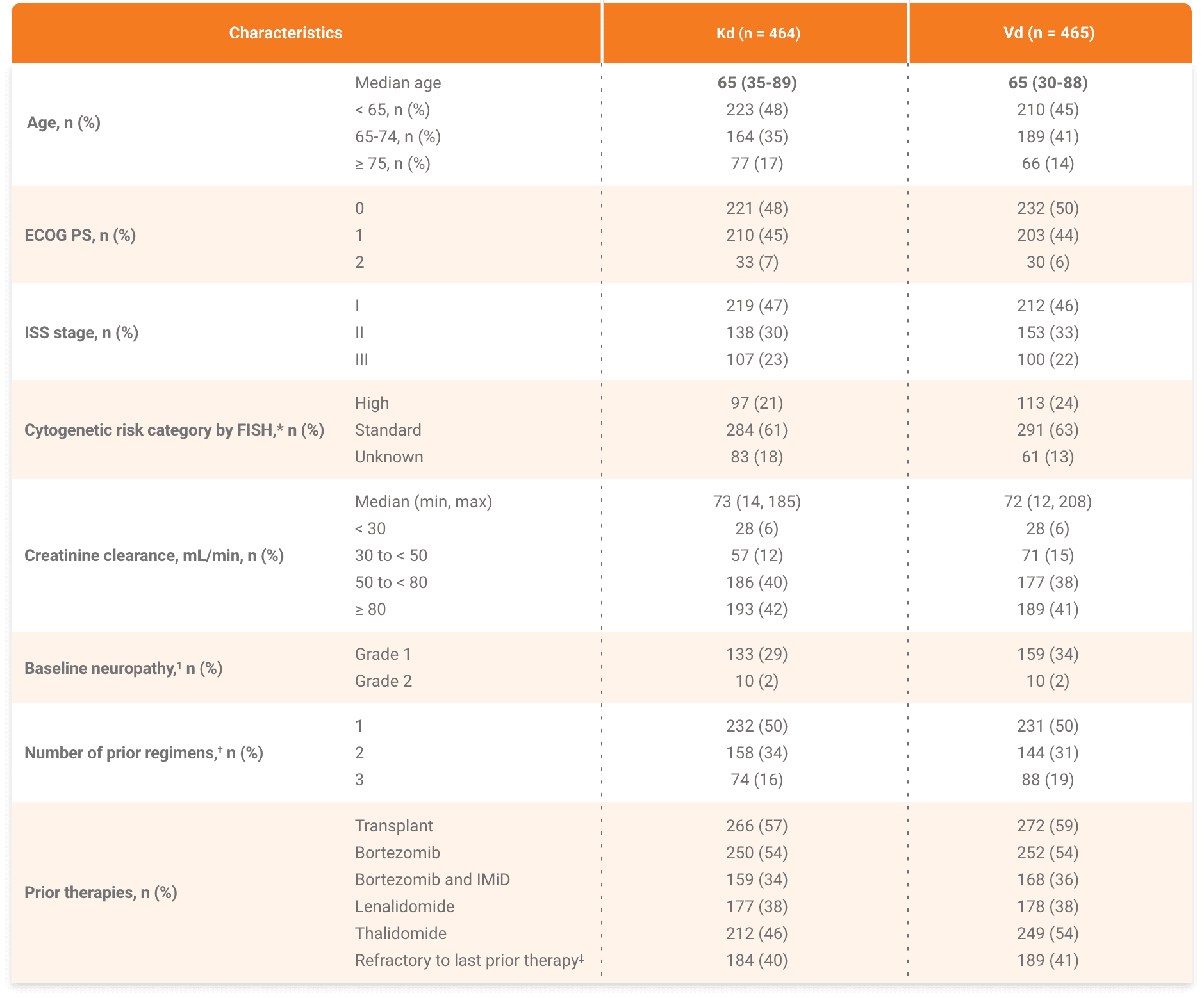
*The high-risk group consisted of patients with the genetic subtypes t(4;14) or t(14;16) in 10% or more of screened plasma cells, or deletion 17p in 20% or more of screened plasma cells based on central review of bone marrow samples obtained at study entry. The standard-risk group consisted of patients without these genetic subtypes.1
†Two patients in the Vd arm had four prior regimens.2
‡Refractory = disease not achieving a minimal response or better, progressing during therapy, or progressing within 60 days after completion of therapy.2
IMiD, immunomodulatory agent; ISS, International Staging System.
Kd, carfilzomib + dexamethasone.
Please see accompanying full Prescribing Information.
Please see accompanying full Prescribing Information.
References: 1. Dimopoulos MA, Moreau P, Palumbo A, et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomized, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17:27-38. 2. KYPROLIS® (carfilzomib) prescribing information, Onyx Pharmaceuticals Inc., an Amgen Inc. subsidiary.