- KYPROLIS® (carfilzomib) is indicated in combination with dexamethasone, or with lenalidomide plus dexamethasone, or with daratumumab plus dexamethasone, ... Read More Close
Meet your patients’ needs with flexible dosing options
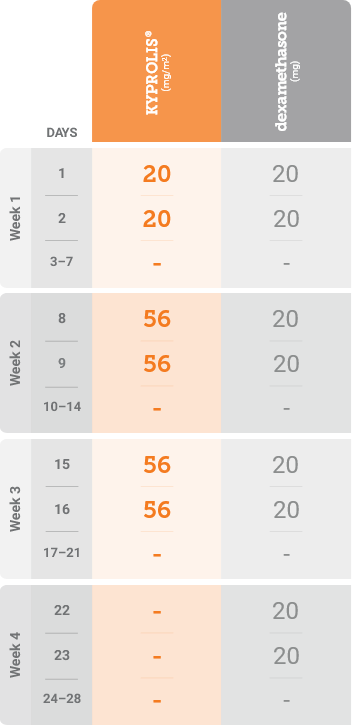
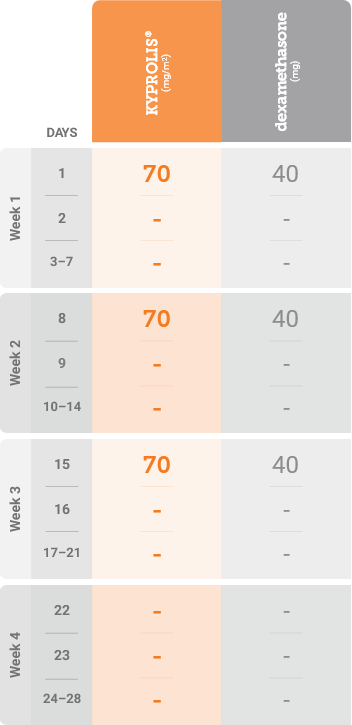
Phase 3, randomized, open-label, multicenter trial (n = 478) comparing KYPROLIS® 70 mg/m2 once weekly plus dexamethasone to KYPROLIS® 27 mg/m2 twice weekly plus dexamethasone in patients with relapsed and refractory multiple myeloma who had received 2 to 3 prior lines of therapy. 478 patients were randomized 1:1 to receive KYPROLIS® 70 mg/m2 once weekly plus dexamethasone (n = 240) or KYPROLIS® 27 mg/m2 twice weekly plus dexamethasone (n = 238) for 28-day cycles until disease progression or unacceptable toxicity. The primary endpoint was PFS. Secondary endpoints included ORR, OS, and safety.2,*
Grade ≥ 3 adverse reactions of interest, Kd 70 mg/m2 once weekly (n = 238) vs Kd 27 mg/m2 twice weekly (n = 235): Peripheral neuropathy (0% vs <1%); acute renal failure (4% vs 6%); cardiac failure (3% vs 4%); ischemic heart disease (1% vs 1%); pulmonary hypertension (0% vs <1%).2
*Kd 27 mg/m2 twice weekly is not an FDA-approved dosing regimen.
FDA, Food and Drug Administration; Kd, carfilzomib + dexamethasone; ORR, overall response rate; OS, overall survival; PFS, progression-free survival.
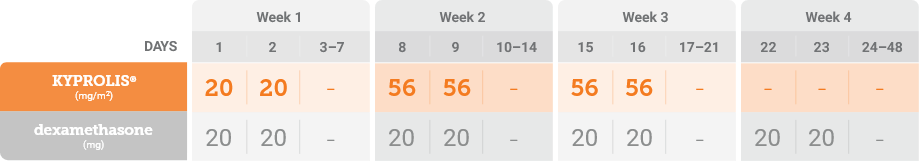
*Until disease progression or unacceptable toxicity.
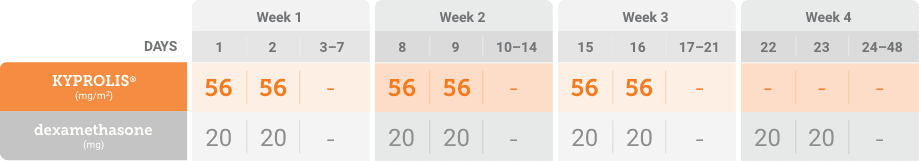
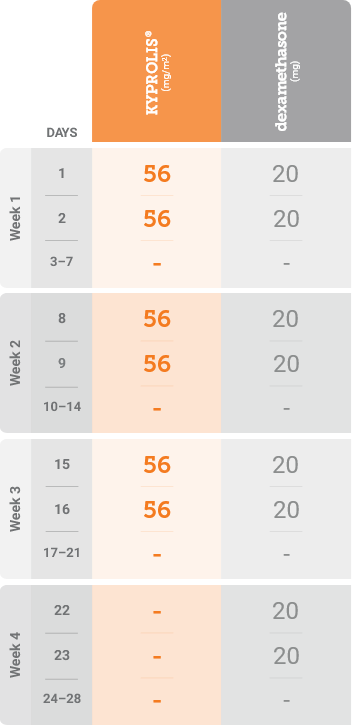
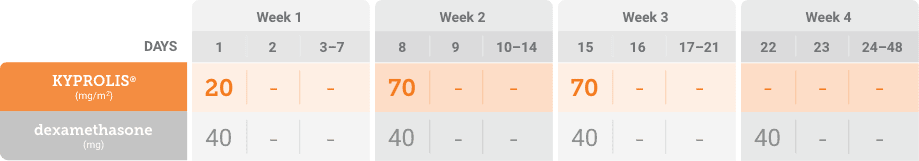
Kd, carfilzomib + dexamethasone.
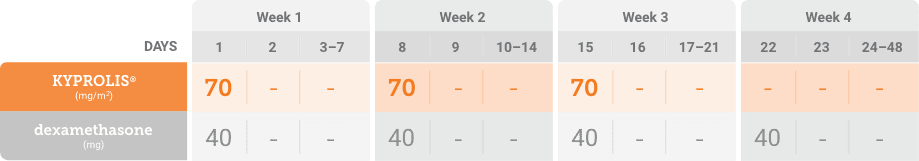

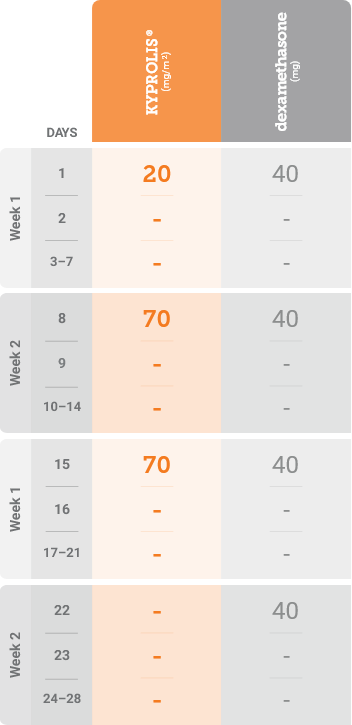
Kd, carfilzomib + dexamethasone.
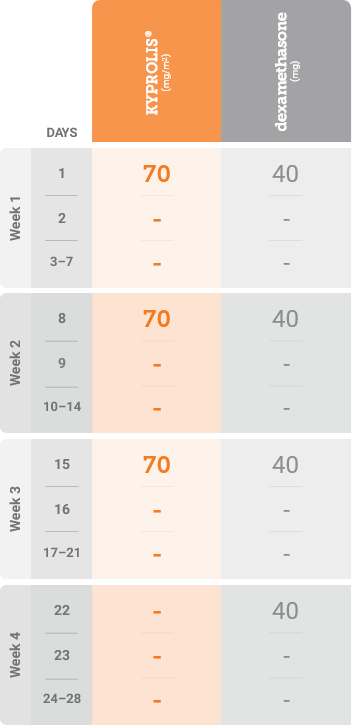
Please see accompanying full Prescribing Information.
Please see accompanying full Prescribing Information.
References: 1. KYPROLIS® (carfilzomib) prescribing information, Onyx Pharmaceuticals Inc., an Amgen Inc. subsidiary. 2. Moreau P, Mateos M-V, Berenson JR, et al. Once weekly versus twice weekly carfilzomib dosing in patients with relapsed and refractory multiple myeloma (A.R.R.O.W.): interim analysis results of a randomized, phase 3 study. Lancet Oncol. 2018;19:953-964.